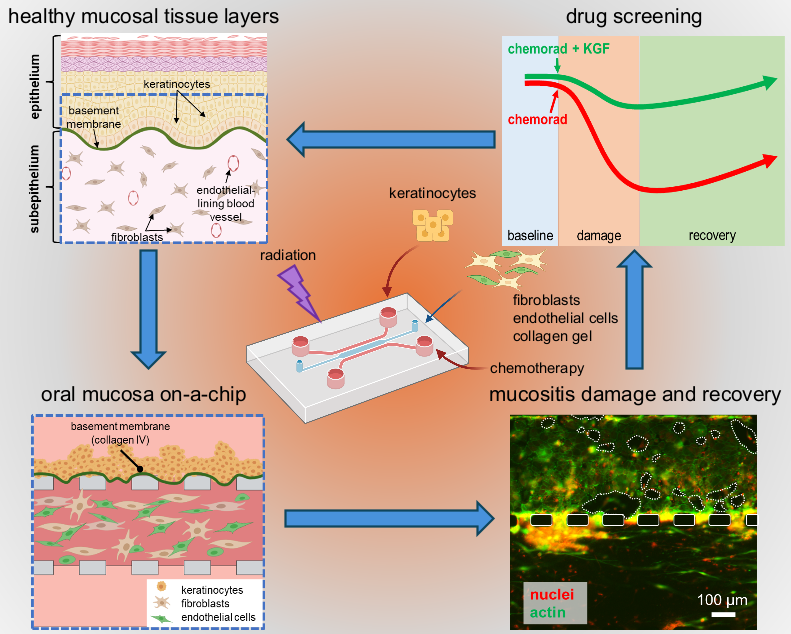
Researchers in the School of Engineering at The Catholic University of America have created a novel tissue chip to study oral mucositis, a painful condition involving extensive mouth ulcers, in cancer patients treated with chemo- and radiotherapy. Tissue chips feature micro-scale channels in an elastomeric material—about the size of a microscope slide—hosting human cells cultured in tissue-like configurations. In a recent paper published in Advanced Healthcare Materials, authors Khanh Ly, Ph.D. (first author, Biomedical Engineering), May Rajtboriraks (undergraduate, Biomedical Engineering), Ahmed Elgerbi (Ph.D. candidate, Biology), Xiaolong Luo, Ph.D. (Mechanical Engineering) and Chris Raub, Ph.D. (corresponding author, Biomedical Engineering) determined cell and molecular responses of gingival cells in the chip to cisplatin (a chemotherapy drug) and to ionizing radiation, as well as demonstrated protection of cells in the chip by palifermin, the only mucositis drug approved by the FDA.
Oral mucositis affects up to half of cancer patients treated with conventional chemotherapy and radiation therapy. It can significantly impact a patient’s quality of life by causing pain and difficulty eating, speaking, and swallowing. Furthermore, infections associated with oral mucositis ulcers can lead to delays in cancer treatment, prolonged hospitalization, and even death. Palifermin, a modified human growth factor, is the only drug approved by the FDA to treat oral mucositis. Palifermin is only approved for about 4% of cancer patients undergoing whole body radiation and stem cell transplantation to treat certain blood cancers. In the past decade, several promising mucositis drug candidates have failed to earn FDA approval after expensive clinical trials.
The oral mucositis tissue chip developed by this team holds promise in the search for safe and effective anti-mucositis therapies. As a simple, inexpensive, yet reliable platform to study cancer-treatment-induced mucositis, it allows researchers to directly observe damage and recovery of the cells in the mucosa-like tissue layers in the chip, using microscopes. At the same time, sensitive molecular assays probe the status of cells in the chip by measuring cell death and the levels of pro-inflammatory proteins secreted by the cells into nutrient media within the tiny microfluidic channels. Corresponding author, Dr. Christopher Raub noted, “In the future, tissue chips may prove to be an important pre-clinical step in the drug development pipeline, by testing lead compounds in conjunction with animal models to better predict which putative therapies are likely to be successful in clinical testing. They may also reduce the total number of animals required for pre-clinical testing and improve the 10% success rate of clinical trials to bring therapies to market.”
Several findings using the mucositis chip demonstrated its clinical relevance and utility for drug testing. First author Khanh Ly noted, “The microfluidic oral mucositis model showed that cell death in response to cisplatin occurred earlier in the sub-epithelium, the tissue layer that is deeper in the oral mucosa than the surface layer called the epithelium. Conversely, radiation treatment first affected the epithelial layer, which has more rapid cell division.” These effects confirm findings published previously by the same group in the journal Biofabrication. The effects of palifermin applied to the chip just before and after the cancer treatments were not previously reported, however, “The protective effects of palifermin extended to keratinocytes and subepithelial cells, mitigating the severity of damage and accelerating functional recovery of mucosa tissue in the chip,” said Dr. Ly. The authors screened pro-inflammatory cytokines secreted into the chip channels and found that palifermin reduced levels of some of these proteins, notably interleukin-8 and tumor necrosis factor-alpha, while other pro-inflammatory proteins remained elevated in the nutrient media bathing cells in the chip. This is notable as it may indicate ways in which palifermin works to reduce some (but not other) inflammatory signals related to mucositis.
Several innovations in this study suggest avenues for further research. “While we will seek to validate screening of pro-inflammatory proteins found in the chip with quantitative molecular assays, the possibility of combination mucositis therapies working synergistically at the molecular level is a hypothesis we will test in the mucositis tissue chip”, said Raub. He also added, “...the relatively long life-time of the mucositis tissue chip, which we cultured for 18 days, suggests that it may be possible to match clinical or preclinical treatment schedules in the tissue chip to more accurately represent the time course of mucositis damage and recovery.” Previous mucosa chips failed as effective models of mucositis, because they did not last long enough in culture before the cells died or grew out of control. To solve this, the researchers implemented a novel in-chip chemical reaction, initiated by blue light, that preserved epithelium and sub-epithelium layers by introducing molecular bonds in the tissue construct to strengthen it without killing the cells in the construct, effects that last for up to three weeks.
Ultimately, for tissue chip models to become useful in drug development, they should maintain consistency across tests and time, and should be validated with pre-clinical and clinical results. Drug industry standard in vitro tests must also be high-throughput, allowing at least 96 tests in parallel, so that several drugs can be tested over a range of doses, singly and in combination, at the same time. Further research and innovations in tissue chip design will be needed for this technology to be useful in drug development. However, the technology will be used immediately to uncover details about molecular responses of the oral mucosa to cancer treatments and putative mucositis treatments, which could open up new avenues for treatment of this harmful side effect of cancer therapies.
